Liquid biopsies, a cutting-edge diagnostic tool, are changing the landscape of cancer detection. They harness bodily fluids, such as blood, to identify a range of cancers by scrutinizing specific biomarkers. These tests zero in on disease-related proteins, circulating tumor cells (CTC), tumor-secreted extracellular vesicles (EVs) carrying biomolecules, and circulating tumor ctDNA using advanced sequencing techniques.
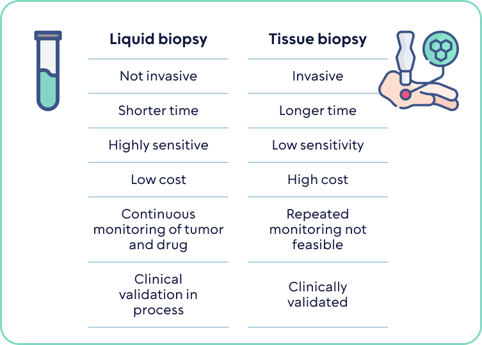
The primary challenge in developing liquid biopsies lies in achieving high sensitivity and specificity to make these tests clinically valuable. While the initial chapter of liquid biopsies concentrated on guiding treatment decisions, the next phase is geared towards detecting early-stage disease and establishing liquid biopsies as the standard of care.
The advantages for patients are significant. Late-stage cancer diagnoses come with a dismal five-year survival rate of just 21%. Conversely, detecting cancer at a localized stage, before it spreads, increases the survival rate to a remarkable 89%. Liquid biopsies have the potential to detect multiple cancer types in a single test, offering the possibility of routine screening and early intervention.
Though the therapy selection market is already established and liquid biopsies are used alongside tissue biopsies, they are starting to move into larger markets - first to assess whether cancers have returned through minimal residual disease (15m patients in the US) and second to move truly into early disease detection (120m average risk individuals). Together liquid biopsies are estimated by one of the major players to be a $55bn market1.
Read more about liquid biopsies, the companies leading the charge in this transformative field, and other revolutionary oncology innovations in our latest report/


 Back
Back

.png)