Keytruda (Pembrolizumab) is the world’s biggest drug by sales, on track for more that $24bn in sales in 20231. The drug targets programmed cell death protein 1 (PD-1) receptor, a so-called immune checkpoint. This target was a breakthrough in the field of immuno-oncology, turning the body’s own immune system against cancer by switching off the chemical brakes that cancers use to evade it. Initial clinical studies showed an impressive response rate of 20%. In some setting and in some patients these drugs showed survival curves that didn’t reach zero i.e. a cure. PD-1s were the early winners in the space moving drugs like Keytruda to standard of care for multiple cancers and the agent of choice for combinations.
However, even with such high response rates there was significant room for improvement and many rushed to study this rapidly emerging field. Unfortunately, subsequent efforts to find new checkpoint inhibitors as well as targets that accelerate the immune systems activity, to build on PD-1s, have largely failed, putting a chill on the space.
We think a newfound interest will emerge in immuno-oncology space. This relates to work on targets like:
Catalyst 1 – Skyscraper-01 study
In Q1 2024, we could see the first piece of evidence of these new approaches with the full readout of the much-expected Phase III Skyscraper-01 study which is testing Roche’s tiragolumab, a TIGIT antibody. The readout will focus on overall survival, the gold standard endpoint for oncology trials, though one that takes some time to mature. Interestingly, some of this data was inadvertently leaked earlier this year, though the analysis was not mature. It showed a positive effect at an interim stage confirming initial clinical data from earlier trials that show extending survival curves (see chart2).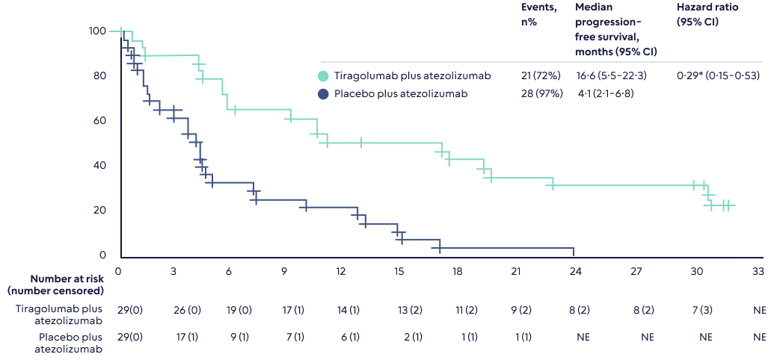
A statistically significant final read out would ignite interest in not just others working on this target, but also the immune-oncology space in general. Different immune targets work synergistically, and the hope is a dual blockade of TIGIT and PD-1 could work well, especially in, as is hypothesized, anti-PD-1 resistant tumor models. TIGIT is also unique in that it enhances the activity of two types of immune cells - not only anti-tumor effector T-cells but also NK-cell responses.
Catalyst 2– LAG3
Also in 2024 the Phase II study by Bristol-Myers of a combination of their PD-1 inhibitor (novlumab), relatimab (a LAG3 antibody), and chemotherapy is due to read out. This is a study in front line non-small cell lung cancer (NSCLC), one of the most important cancers.
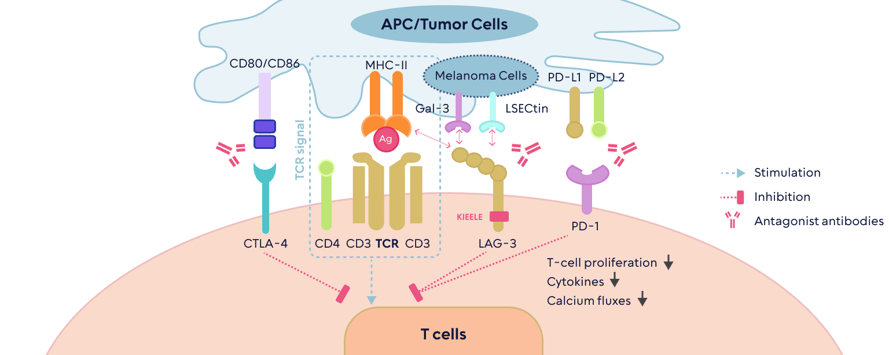
LAG3 is very similar to PD1, as tumors use this molecule to downregulate the activity of the immune system. This means that blocking it could make tumors visible to the immune response. LAG3 is expressed in many cancers and is associated with a bad prognosis. A positive read out in this trial sets the stage for several important companies working on the LAG3 target.
Why all this matters
Harnessing the immune system is a biologically sound strategy, as it has the greatest potential for specific destruction of tumors, reduced toxicity to healthy tissues and the potential for memory to prevent recurrence. Yet since the success of PD-1s little clinical progress has been made and many investors have given up on the immuno-oncology space. We believe that this is about to change. For many established immune-oncology players adding new molecules has the potential to extend the lifecycle of their existing drugs in this area.


 Back
Back

.png)