In oncology, cell therapy is a groundbreaking approach that involves using engineered immune cells, often sourced from the patient, as a "living drug" to combat cancer. Typically, T-cells and natural killer (NK) cells are employed, and they can be enhanced through gene editing techniques such as CRISPR. While the technology has gained approval in hematological cancers using autologous cells, the next horizon involves targeting solid tumors and using off-the-shelf or allogeneic cells. Challenges include manufacturing complexities and issues related to the tumor microenvironment.
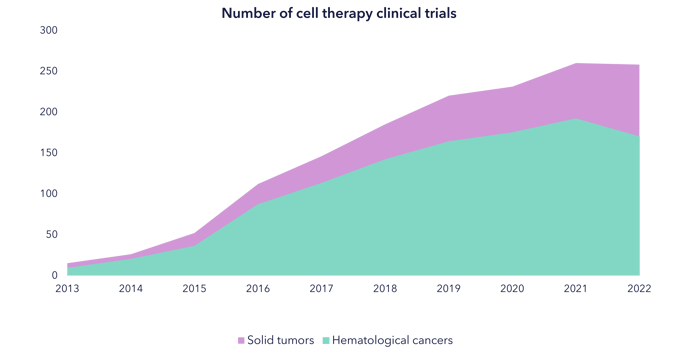
The impact of cell therapies in oncology has been profound. Since 2017, the FDA has approved six CAR-T therapies for blood cancers, with clinical trials showing impressive overall response rates (ORRs). A single infusion can have a long-lasting, potentially curative effect. However, the high cost of manufacturing and the personalized nature of these therapies remain challenges.
One promising market to watch is multiple myeloma, the third most common blood cancer, which could be disrupted by cell therapies. Over 170,000 global diagnoses of multiple myeloma provide a substantial potential impact1. Overall, the oncology cell therapy market is expected to grow at a rate of 23% per year from its current value of $8 billion2, primarily due to expansion into solid tumors and the use of off-the-shelf platforms.
Read more about cell therapies, the companies leading the charge in this transformative field, and other revolutionary oncology innovations in our latest report.


 Back
Back

.png)